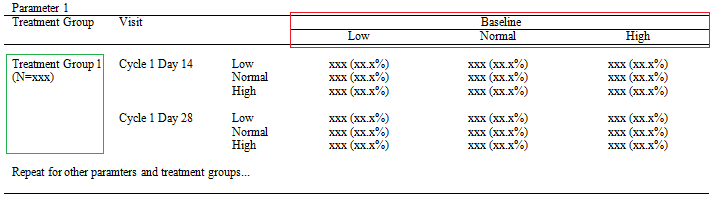
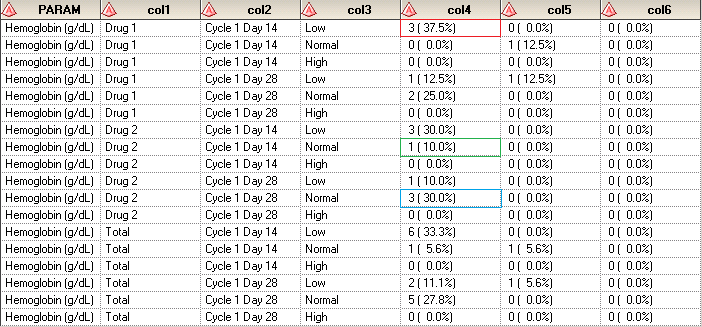
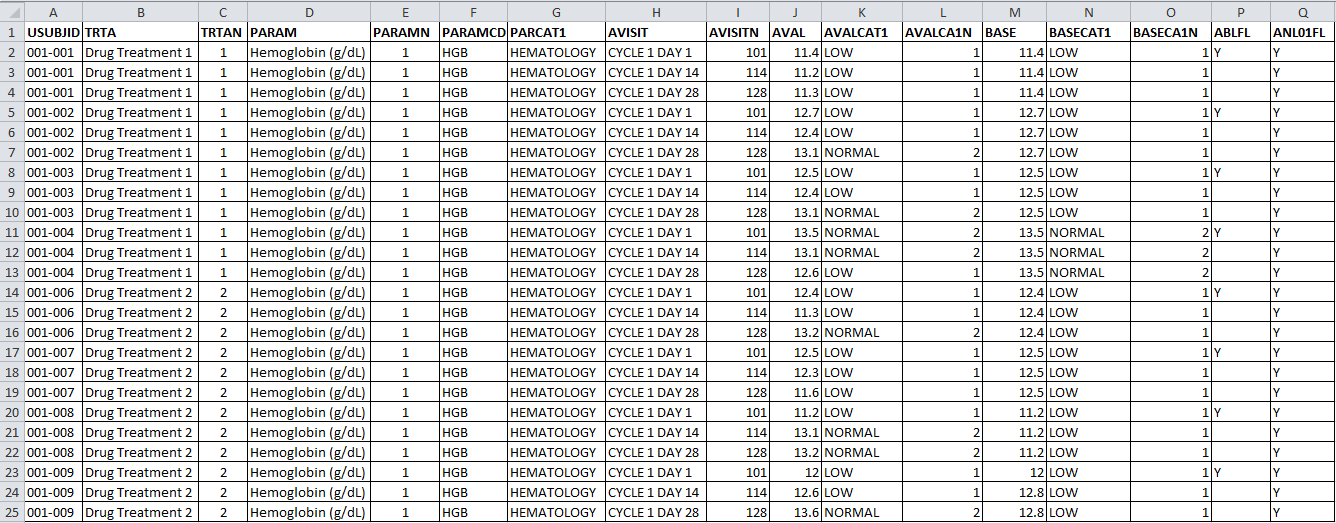
CLINICAL STUDY REPORT - IN-TEXT TABLES, TABLES FIGURES AND GRAPHS, PATIENT AND INDIVIDUAL PATIENT DATA LISTINGS: ICH E3 TECHNIC

Treat to Target by Specific Cytokine Interdiction: Multiple Biomarker Disease Activity Test Deconstructed - ACR Meeting Abstracts

Table 1 from Time to move on from 'time-to-first': should all events be included in the analysis of clinical trials? | Semantic Scholar
Robust multivariate nonparametric tests for detection of two-sample location shift in clinical trials | PLOS ONE
Re-evaluating randomized clinical trials of psychological interventions: Impact of response shift on the interpretation of trial results | PLOS ONE
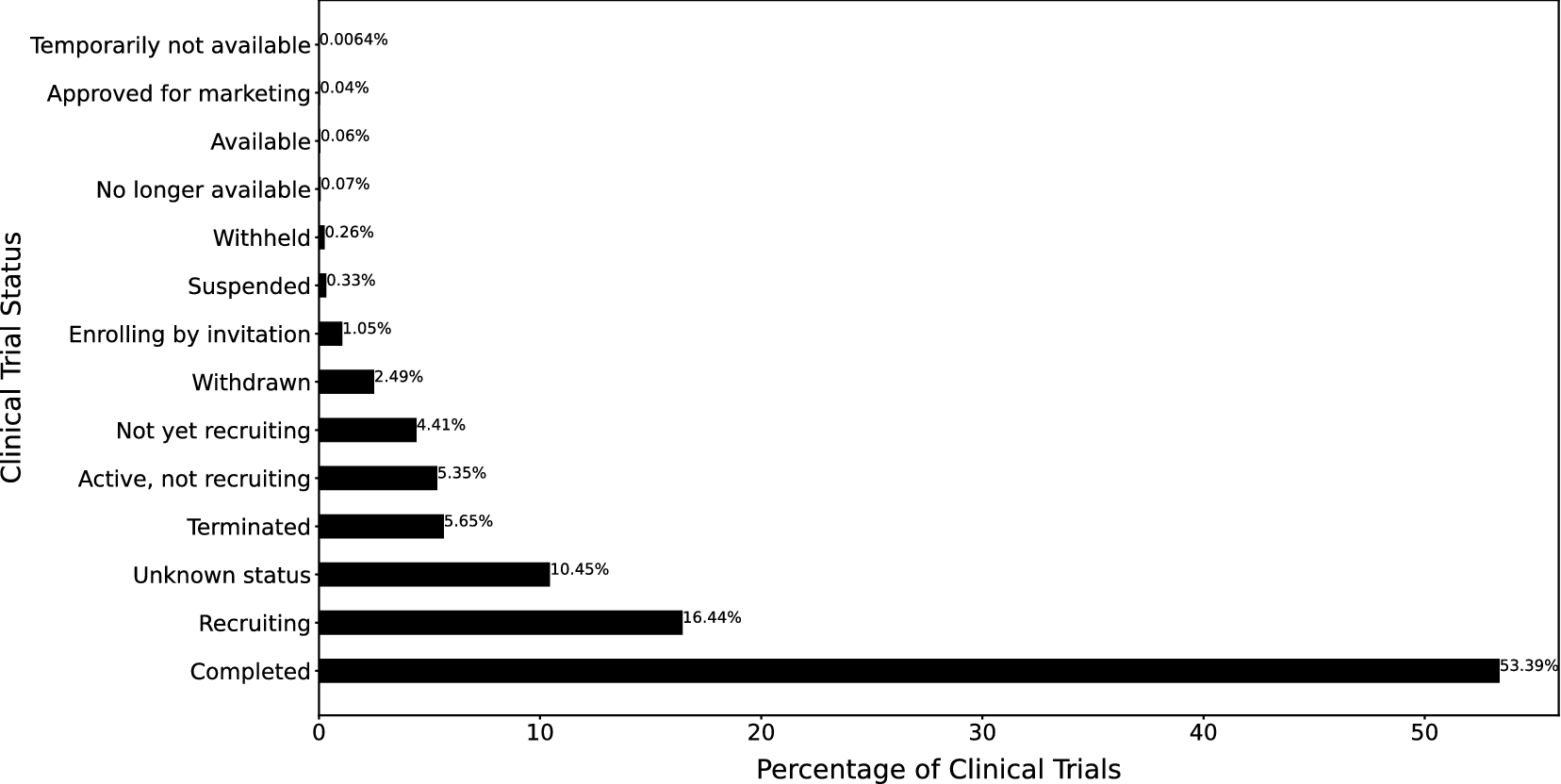
Predictive modeling of clinical trial terminations using feature engineering and embedding learning | Scientific Reports
Robust multivariate nonparametric tests for detection of two-sample location shift in clinical trials | PLOS ONE

The Low‐Risk Profile in Pulmonary Arterial Hypertension. Time for a Paradigm Shift to Goal‐oriented Clinical Trial Endpoints? | Semantic Scholar

Missing outcome data management in acute stroke trials testing iv thrombolytics. Is there risk of bias? - Jose Fernandez-Ferro, Lee H Schwamm, Miguel A Descalzo, Rachael MacIsaac, Patrick D Lyden, Kennedy R