Final Rule Confirms, Posting of Study Results on ClinicalTrials.gov Will be Required for Unapproved Products - IMPACT Pharmaceutical Services, Inc.

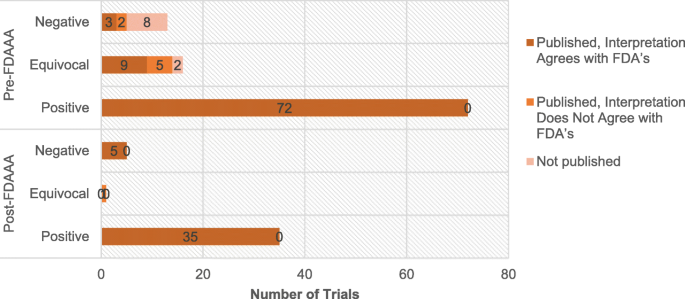
Identification of trials reviewed by the FDA for New Drug Applications... | Download Scientific Diagram
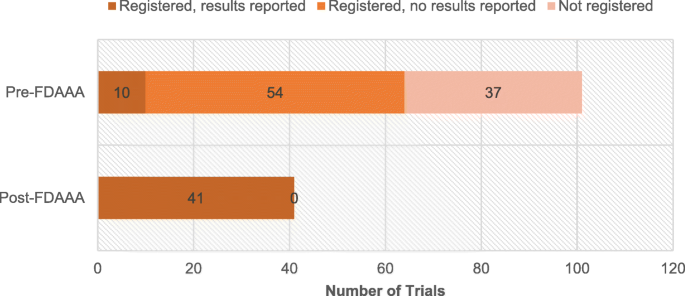
Registration, results reporting, and publication bias of clinical trials supporting FDA approval of neuropsychiatric drugs before and after FDAAA: a retrospective cohort study | Trials | Full Text

PDF) Consistency of trial reporting between ClinicalTrials.gov and corresponding publications: One decade after FDAAA
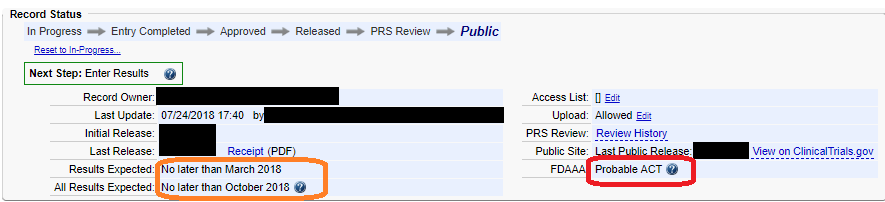
ClinicalTrials.gov Tip of the Week: Seife et al. v. HHS et al.: Results Must be Submitted for “pACTs” to Clinical Trials.gov