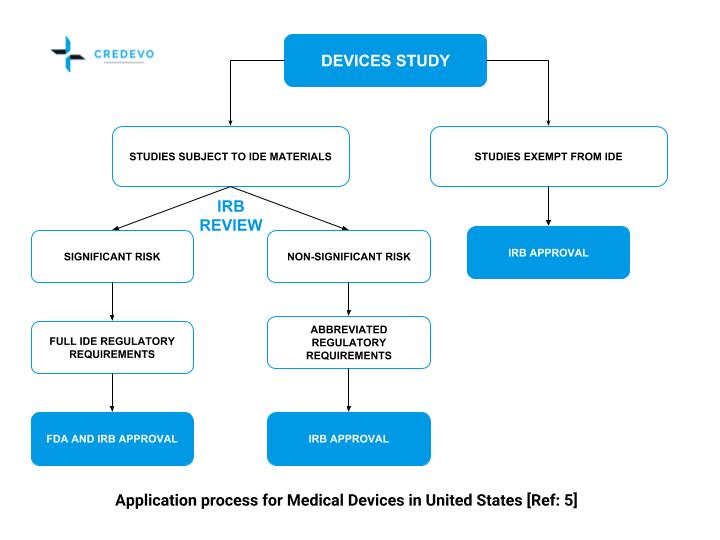
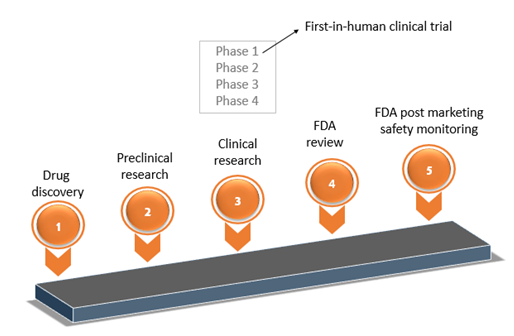
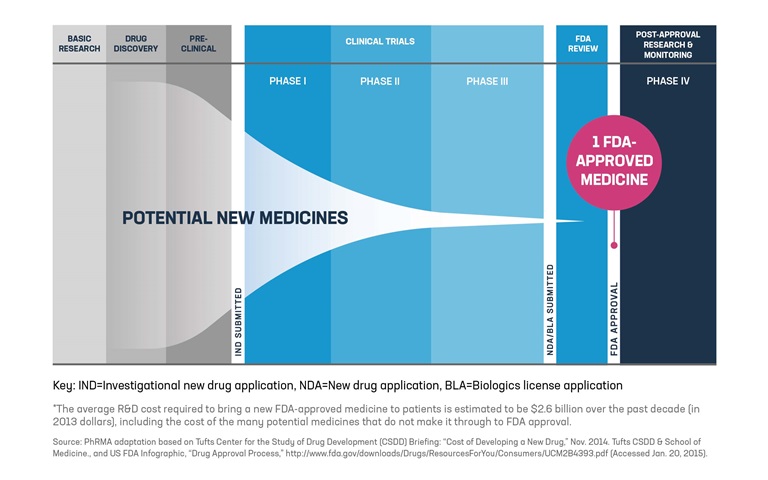
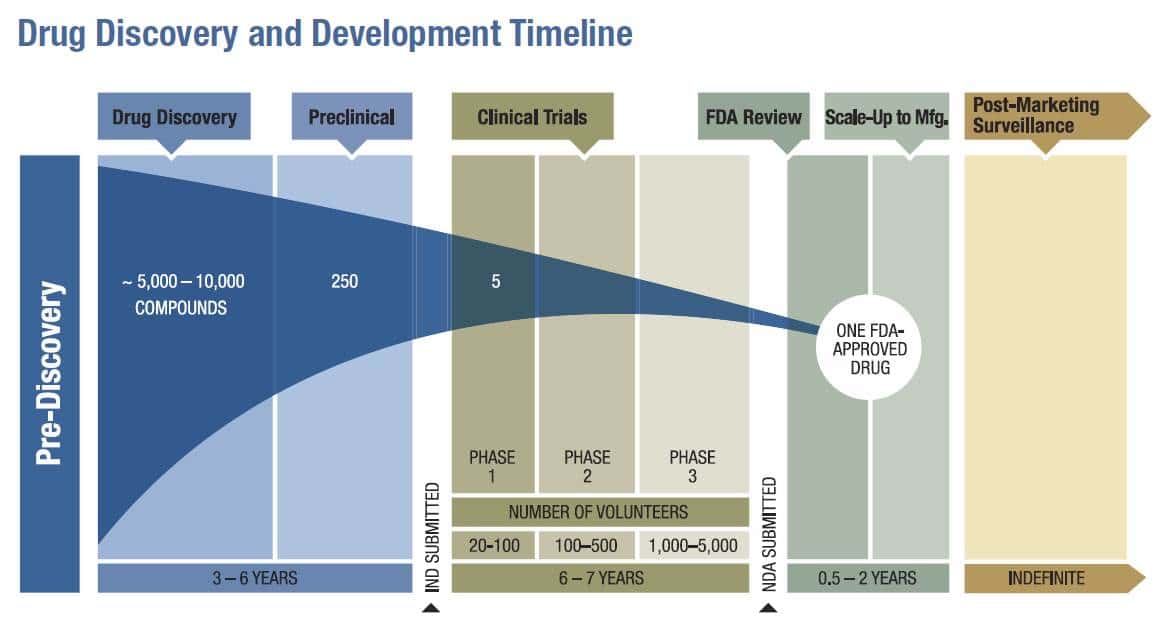
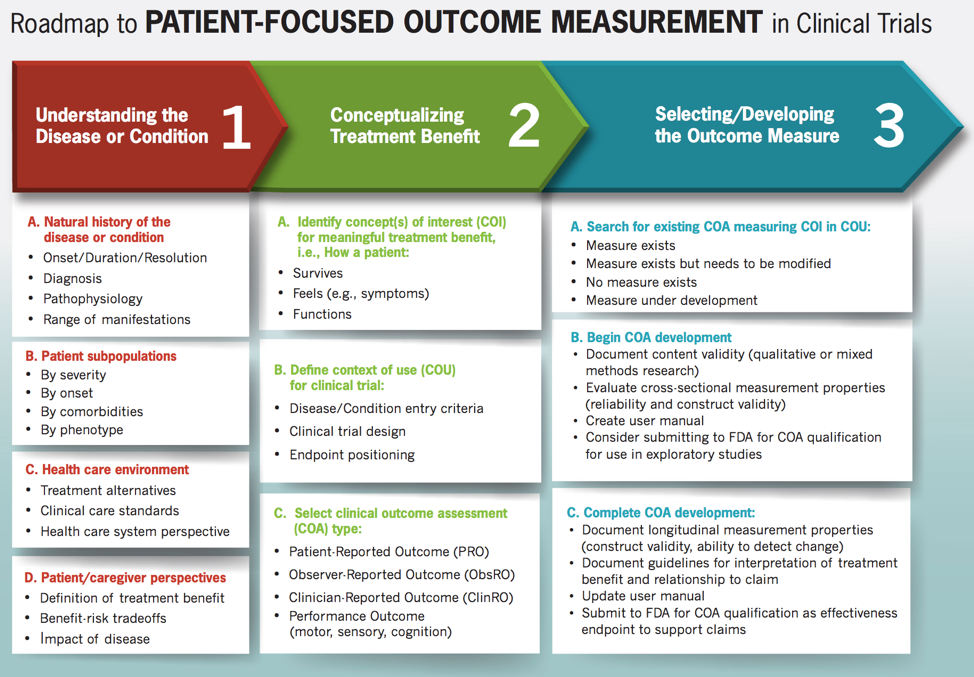
FDA roadmap to patient-focused outcome measurement in clinical trials. 3 | Download Scientific Diagram

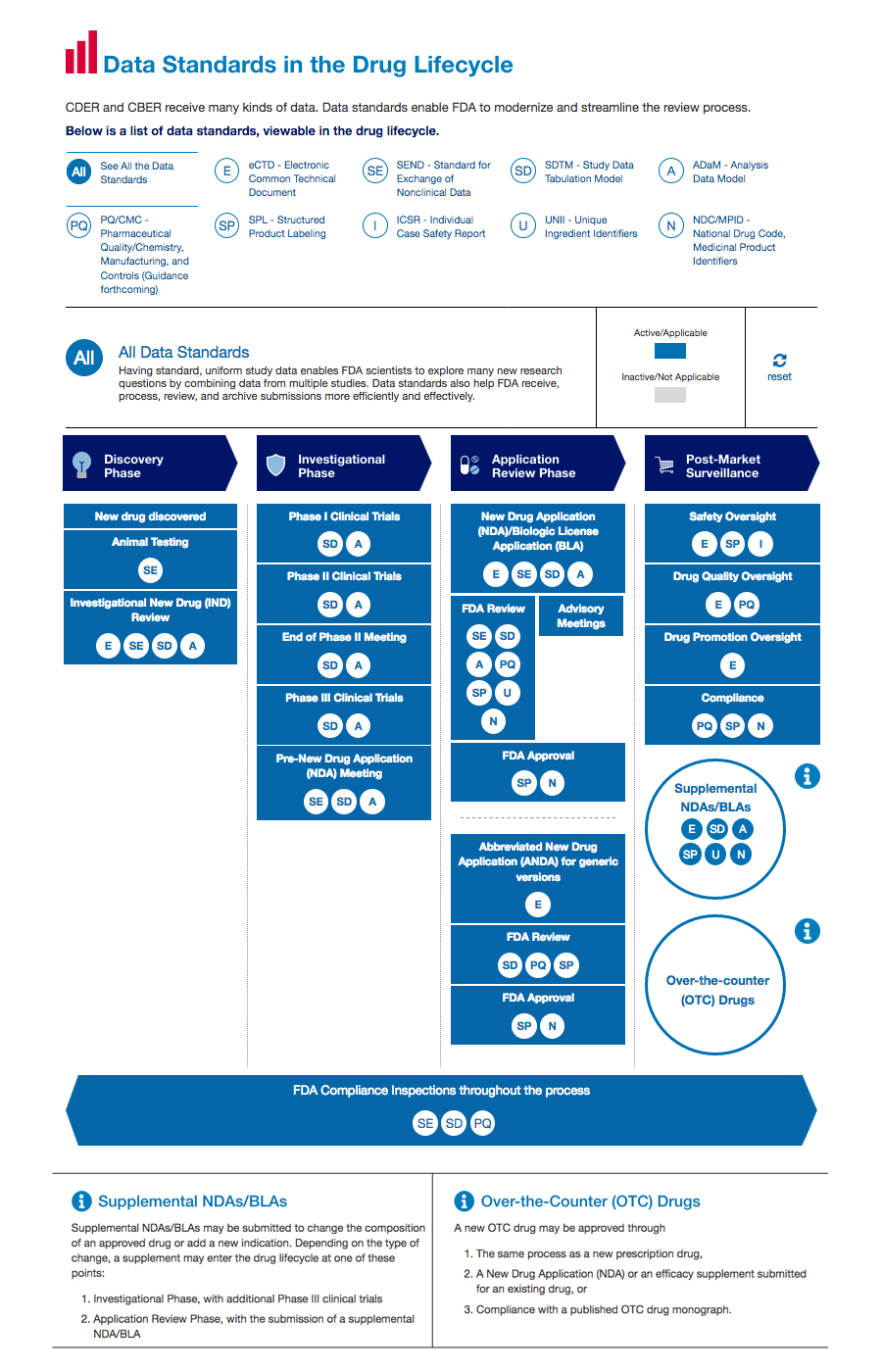
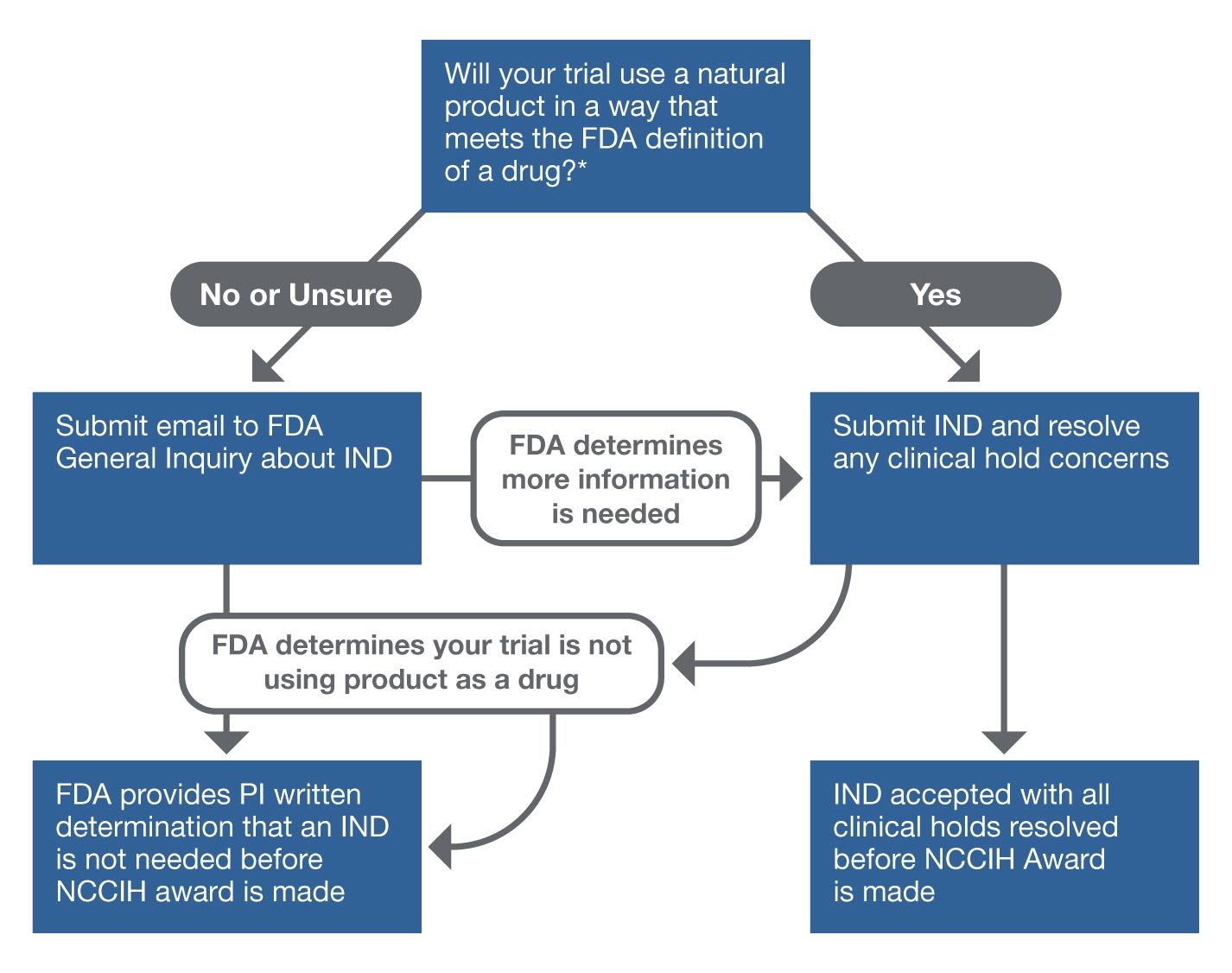
FDA Clears Up Remote Data Use In Clinical Trials With New Guidance | The Healthcare Technology Report.

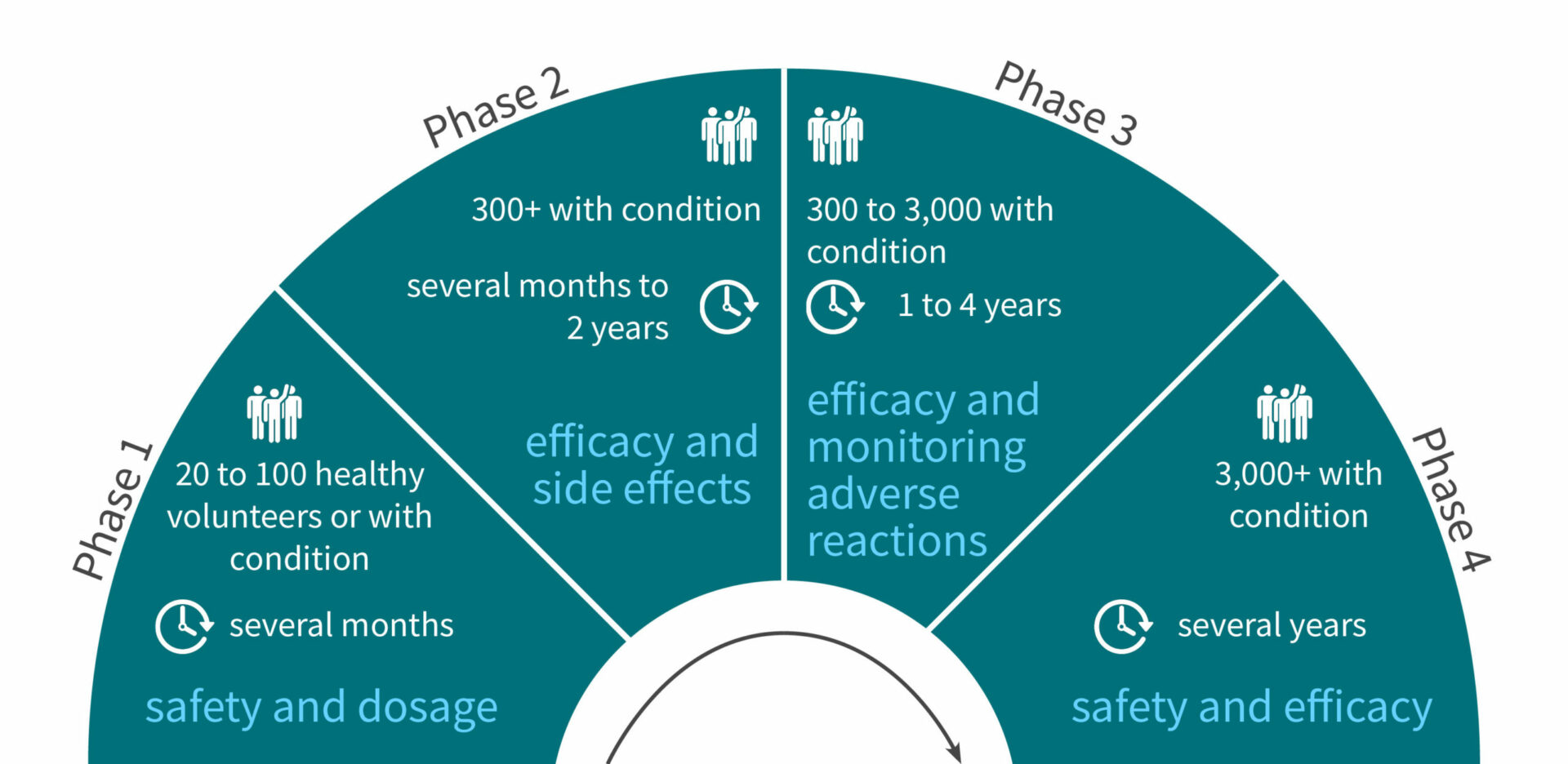
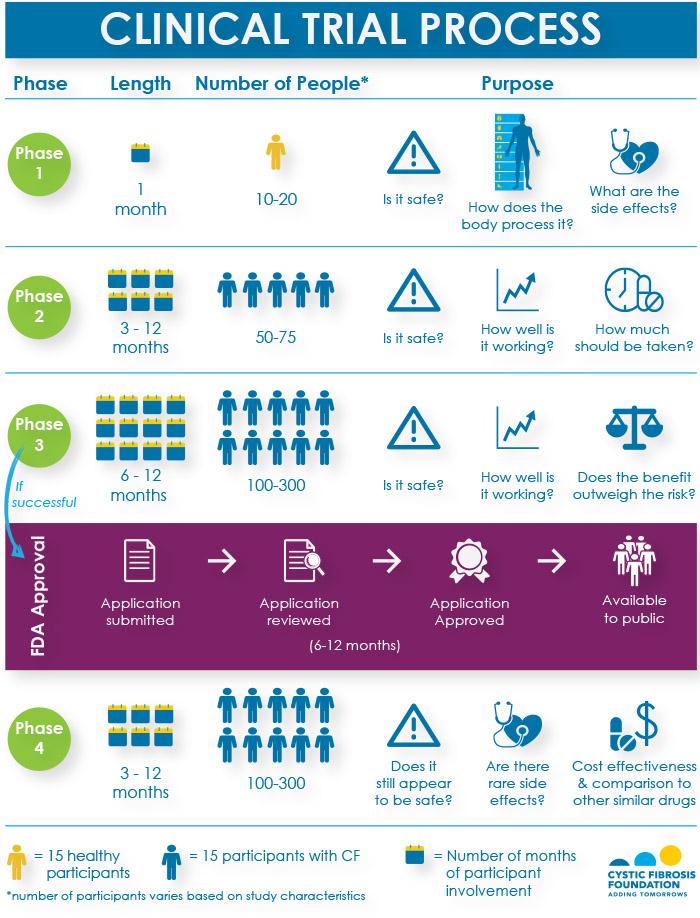
Characteristics of clinical trial phases according to U.S. Food & Drug... | Download Scientific Diagram