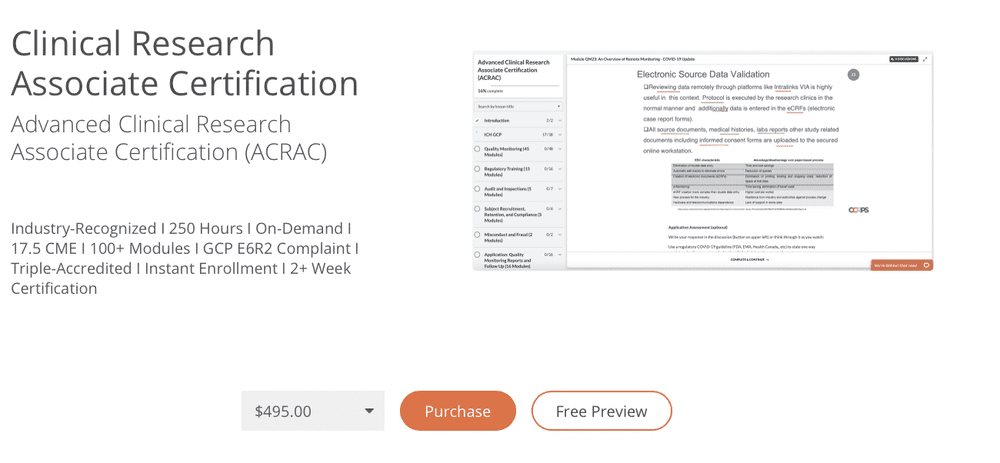
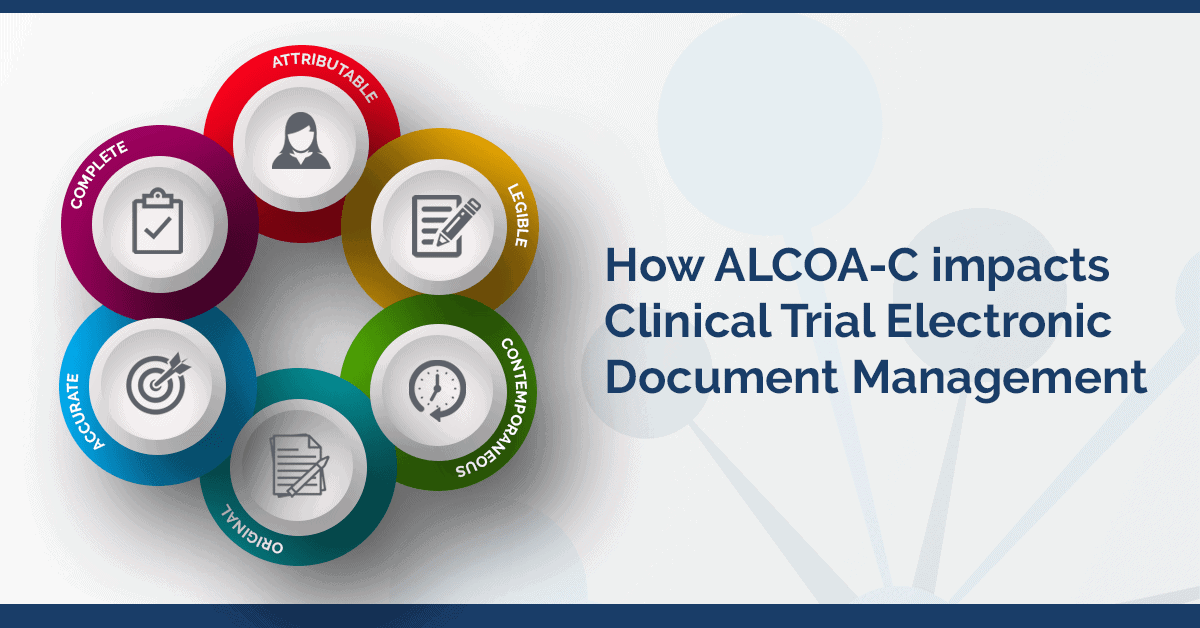
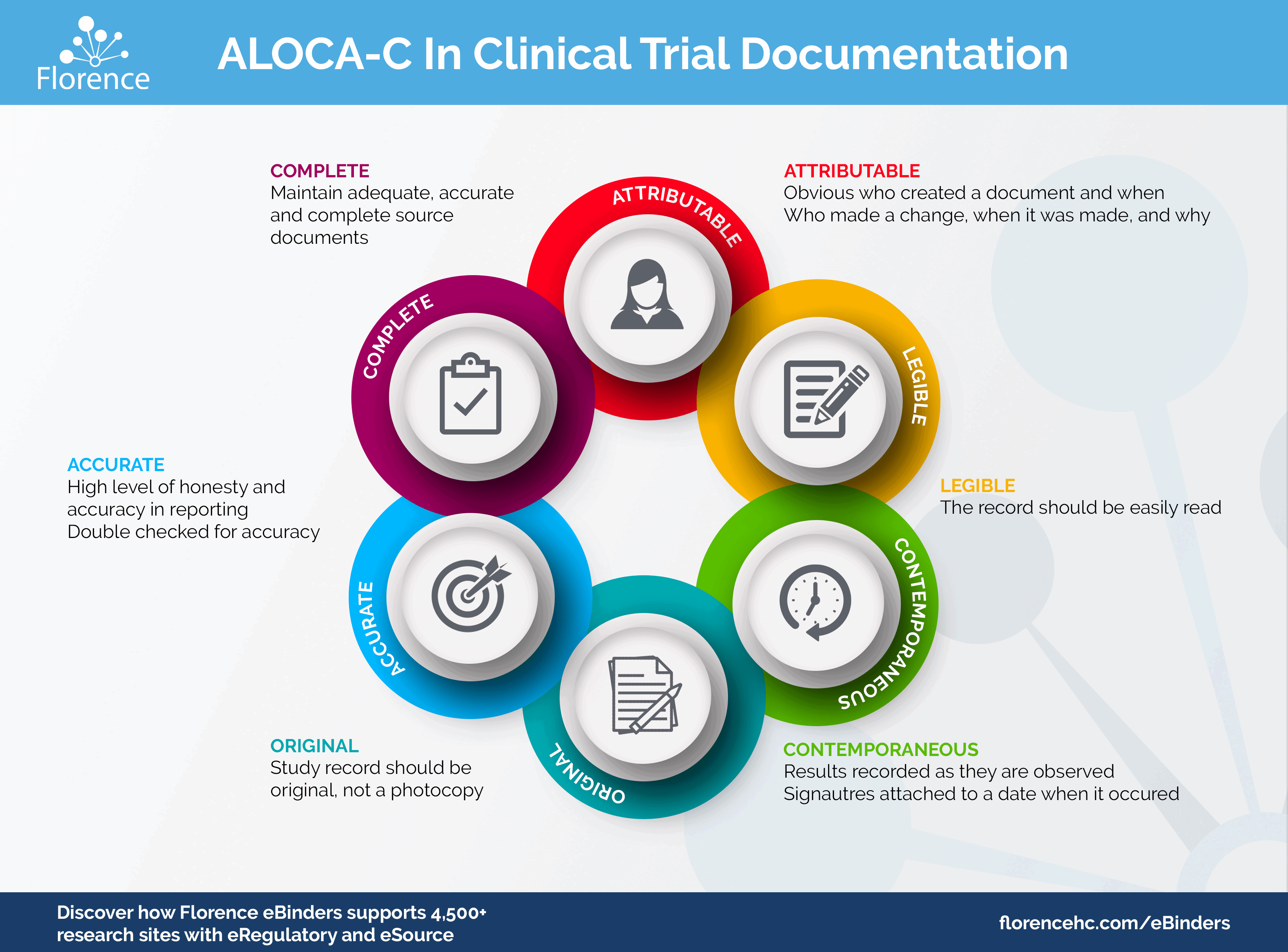
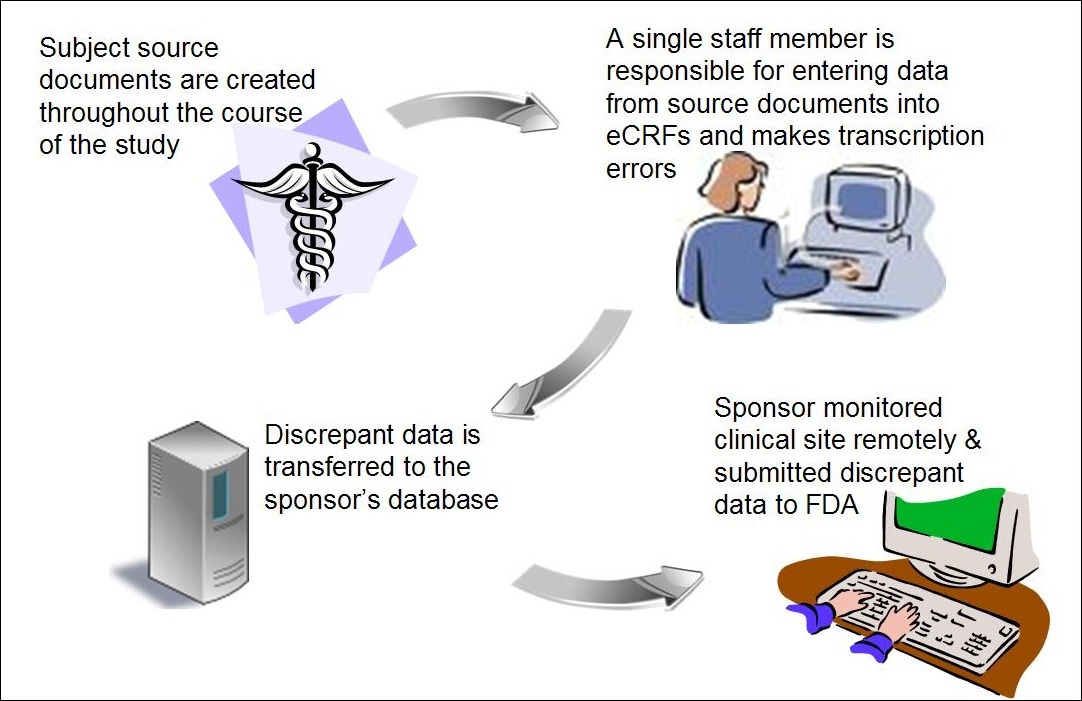
PDF) Research Electronic Data Capture (REDCap) electronic Informed Consent Form (eICF) is compliant and feasible in a clinical research setting
Reflection paper on expectations for electronic source data and data transcribed to elecronic data collection tools in clinical

Data Management for Pharmaceutical Trials Michael A. Kohn, MD, MPP (Acknowledgment: Susanne Prokscha) - ppt download