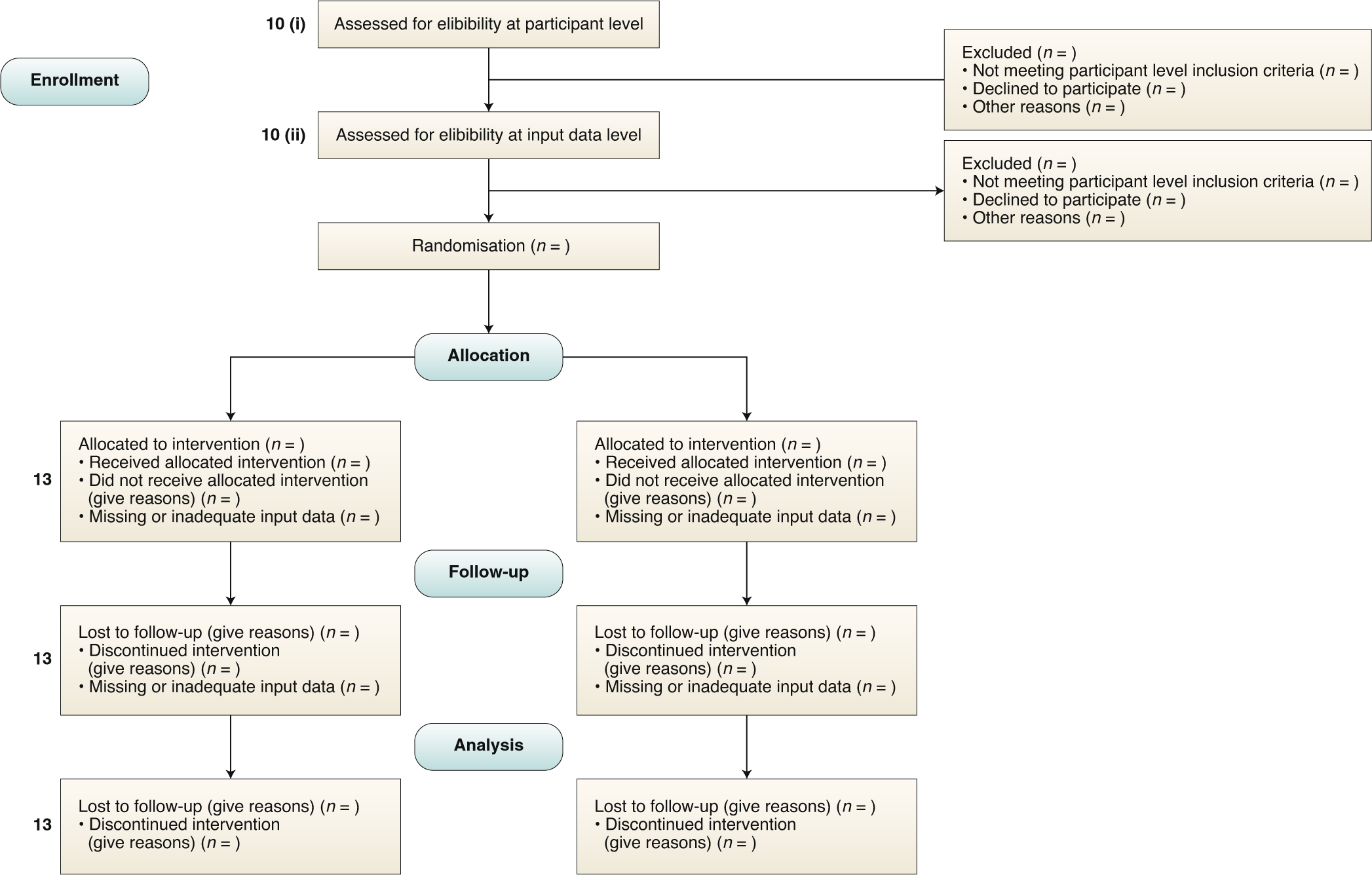
Book 6: 2023 Clinical Trials in The EU: Selected Legislation, Guidelin – Clinical Research Resources, LLC
![PDF] RECENT ADVANCEMENT IN REGULATORY GUIDELINES FOR CLINICAL TRIALS IN USA AND INDIA | Semantic Scholar PDF] RECENT ADVANCEMENT IN REGULATORY GUIDELINES FOR CLINICAL TRIALS IN USA AND INDIA | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/52893bcfc57fecda0c60e34dd1fe46414c867575/9-Figure4-1.png)
PDF] RECENT ADVANCEMENT IN REGULATORY GUIDELINES FOR CLINICAL TRIALS IN USA AND INDIA | Semantic Scholar

Guidelines for cellular and molecular pathology content in clinical trial protocols: the SPIRIT-Path extension - The Lancet Oncology
![PDF] RECENT ADVANCEMENT IN REGULATORY GUIDELINES FOR CLINICAL TRIALS IN USA AND INDIA | Semantic Scholar PDF] RECENT ADVANCEMENT IN REGULATORY GUIDELINES FOR CLINICAL TRIALS IN USA AND INDIA | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/52893bcfc57fecda0c60e34dd1fe46414c867575/14-Table2-1.png)
PDF] RECENT ADVANCEMENT IN REGULATORY GUIDELINES FOR CLINICAL TRIALS IN USA AND INDIA | Semantic Scholar
Clinical Trial Guidelines Ppt Powerpoint Presentation Inspiration Example Introduction | PowerPoint Slides Diagrams | Themes for PPT | Presentations Graphic Ideas

Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension - The Lancet Digital Health

Updated clinical trial reporting guidelines include patient voices to improve trial utility and transparency

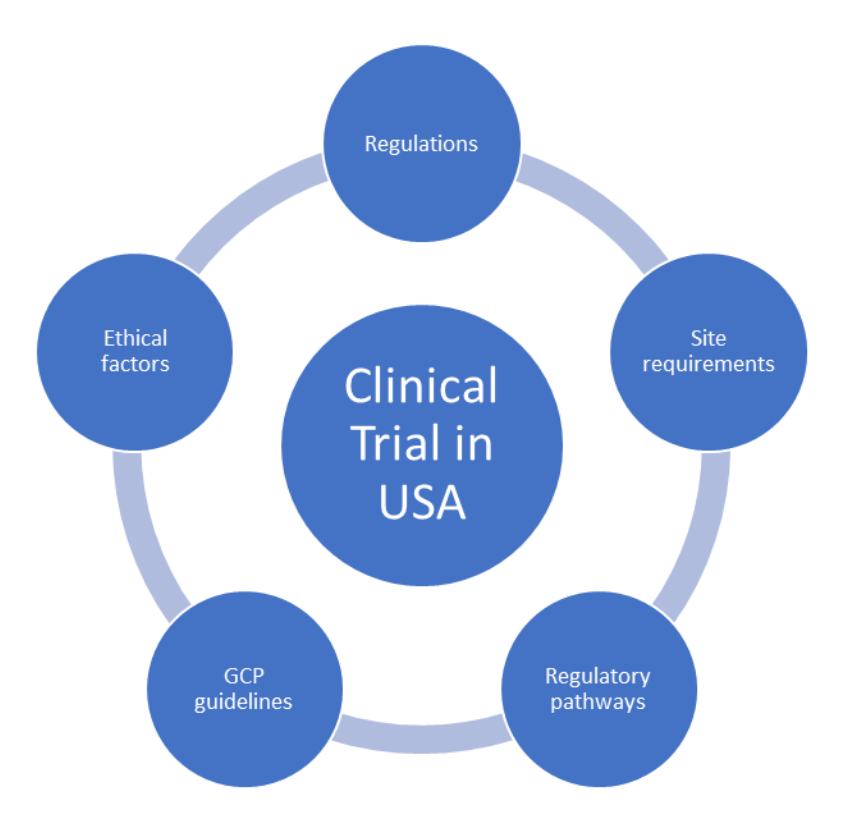
Comparison of clinical trial guidelines in USA, EU and India, Singapore. | Download Scientific Diagram
![PDF] RECENT ADVANCEMENT IN REGULATORY GUIDELINES FOR CLINICAL TRIALS IN USA AND INDIA | Semantic Scholar PDF] RECENT ADVANCEMENT IN REGULATORY GUIDELINES FOR CLINICAL TRIALS IN USA AND INDIA | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/52893bcfc57fecda0c60e34dd1fe46414c867575/2-Figure1-1.png)
PDF] RECENT ADVANCEMENT IN REGULATORY GUIDELINES FOR CLINICAL TRIALS IN USA AND INDIA | Semantic Scholar